Merson Researches Effects of Toxins on Marine Animals
- News & Events
- News
- Merson Researches Effects of Toxins on Marine Animals

Associate Professor of Biology Rebeka Merson and her lab students Keya Thakkar and Timothy Bock Jr.

Environmental contaminants in the marine environment, the adaptation of animals to this environment and climate change are the focus of a research study begun this year by RIC Associate Professor of Biology Rebeka Merson in collaboration with Diane Nacci of the U.S. Environmental Protection Agency (EPA). Merson was awarded a Rhode Island Science and Technology Advisory Council (STAC) grant in February 2015 to fund this project titled, “Narragansett Bay Apex Predators’ Response to Toxic Chemicals and Climate Change.”
Merson is focusing on evolution and toxicology in cartilaginous fish, using as her model the little skate, a fish that is most often used as bait in lobster traps and that has been used as a model organism for biology and medical research.
Merson’s experiment involves exposing little skates to environmental contaminants called polychlorinated biphenyls (PCB) often found in industrial waste. “We are looking for specific developmental changes or deformities that result from exposure to these chemicals,” she said.
“What makes this experiment important is that there is absolutely nothing known about the effects of environmental contaminants on little skates,” she said. “All we know is that little skates are important to the ecosystem and that if they are adversely affected by environmental chemicals, it's likely to impact the ecosystem.”
How these organisms have been affected and how they have adapted to toxic environments are areas of research that Merson has been investigating since her post-doctoral studies at the Woods Hole Oceanographic Institution. The major models she has used in her research are sharks and skates. Little skates, she said, fall within the class of fish that includes sharks, little skates and rays.
“Little skates originate from one of the earliest classes of vertebrates,” she explained. “They grow to a foot-and-a-quarter long and about nine inches wide. They have a skeleton but it’s not made of bone; it’s made of cartilage.” They are bottom-dwellers, feeding on crustaceans, but are preyed upon by sharks and bony fish, note scientists.
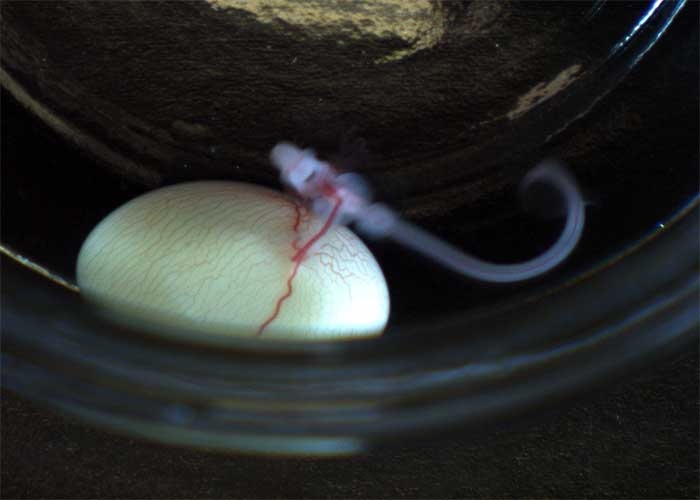
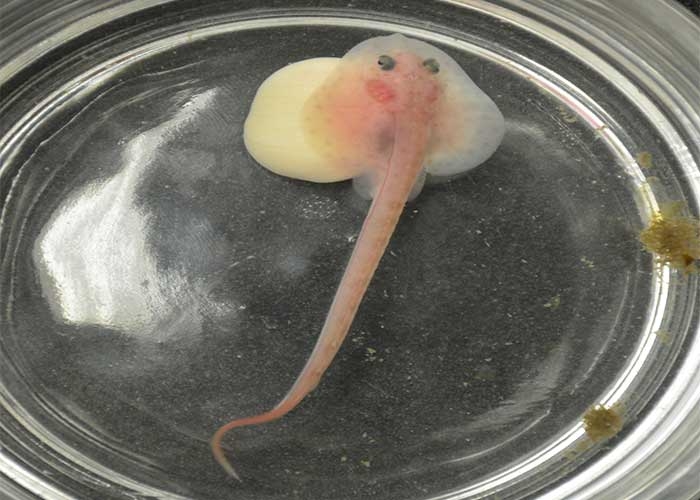
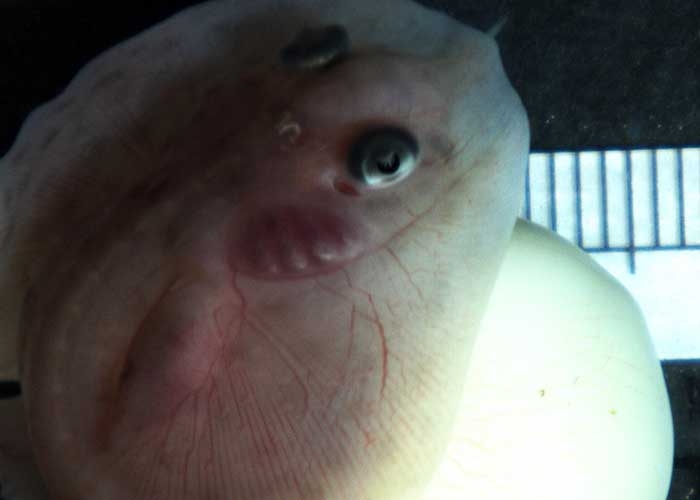
Merson chose the little skate as the focus of her project for a number of reasons. First, because the embryo has a very long developmental period, which allows her research team enough time to study each embryonic stage. “The little skate goes from fertilization to hatching in six to eight months,” she said, “whereas many bony fish used in these studies have a developmental period of only hours.” Secondly, the research team is able to take the embryos out of their egg cases, culture them in seawater and observe them as they develop.
Merson works with seven RIC students: graduate students Timothy Bock and Tomasz Rosadzinski; and undergraduates Sara “Libby” Lazar, Nicholas Andreozzi, Keya Thakkar, Chris Rei-Mohammed and Stephanie Nappa. Two additional research students are expected to join the team in the fall.
The team work out of a research lab at the EPA in Narragansett. Work in the lab began with maintaining adult little skates in a tank at the Rhode Island NSF EPSCoR Marine Science Research Facility at the URI Graduate School of Oceanography. Currently they have seven females who lay three to six egg cases a week. The team then collects the egg cases laid on the bottom of the tank and takes them over to the EPA lab.
The embryos are kept inside their egg case throughout their development, yet the lab team is able to see the embryo inside the case by using what Merson calls a “windowing” procedure. “We scrape off the outer layer of the egg case and, as their growth progresses, we come in and take photos,” she said.
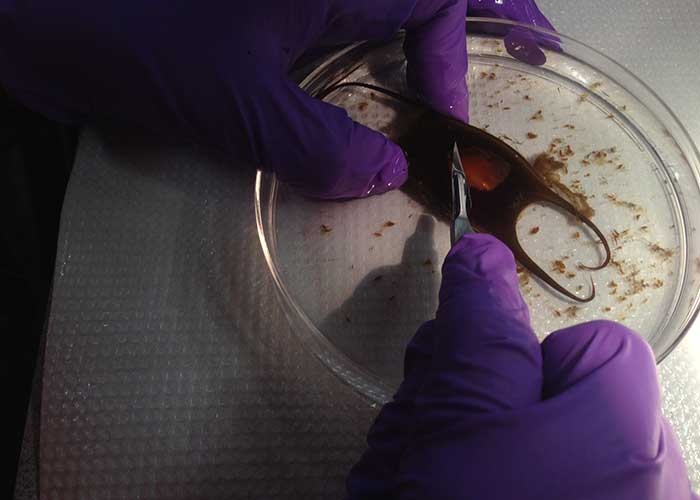
For experiments, the egg case is cut open and the embryos are placed in a 10-gallon tank filled with seawater. The lab houses 10 tanks.
Merson said care is needed to recreate the organism’s natural environment. “We don’t want the experiment itself to affect the results,” she said. Three times a week the team works at the EPA to optimize conditions for experiments, making sure that the water in the tanks maintains the right temperature, salinity and oxygen concentration.
Back at the RIC lab, students isolate DNA and RNA from cells and tissues and learn to use a process called PCR, which allows them to generate thousands to millions of copies of a particular DNA sequence.
When it’s time to expose the embryos to PCB, they will be placed in special experimental containers and the contaminant will be applied to their skin. By using a method called clearing and staining, the team will be able to observe the effects of exposure on skeletal development.
“To clear, we take the specimen and use enzymes to remove the tissue so that you can see through the skin to the organs. Then we use two stains: the blue stain targets the cartilage and the red stain targets the calcified tissue. We have timed exposures because we know [from other studies] that at certain stages of the embryonic period the embryo is more sensitive to toxic effects. The team will take photos at the beginning of the experiment and, a couple of weeks later at the end of toxic exposure,” said Merson. They will be working in a confined area of the EPA. In addition, the EPA can safely dispose of toxic matter used in their experiments.
Exposures to PCBs and related chemicals in other animals have created varied effects, said Merson. “Mice develop cleft palate; bony fish develop cranial facial abnormalities and heart deformities, such as pericardial edema; and in humans, exposure to PCBs impacts fertility” she said.
PCBs and other chemicals are present in Narragansett Bay. According to Merson, the level of contamination is predicted to get worse with climate change. She said, “The increased frequency of heavy precipitation causes sewage treatment plants to overflow, sending waste into the bay. Moreover, runoff from the roads also drain into the bay.
Merson explained how toxic chemicals build up in the bodies of apex predators. “Sharks and rays are what we all apex predators,” she said. “They are at the top of the food chain. Animals at the lower levels of the food chain absorb toxins and are eaten by animals at the next level up, so that those at the top of the food chain have the most contaminants in their system.”
“If animals at the top of the food chain are gone, or if their numbers are severely diminished, then those on the next level down will not be regulated. That level will overpopulate and eat more of the animals at the lower levels. What usually ends up happening is that very few numbers of species flourish and you lose the diversity. So sharks, little skates and rays serve as regulators in this food chain from the top down.”
Merson and Nacci’s STAC grant runs out in May 2016; however, Merson’s research has been funded for many years now by such grant programs as the IDeA Networks of Biomedical Research Excellence (INBRE), the National Institutes of Health (NIH) and the Experimental Program to Stimulate Competitive Research (EPSCoR), which is funded by the National Science Foundation (NSF).
“We’re never finished,” Merson said. “This information will lead us to more questions. That’s always the way it happens. But along the way, we learn more, which we can bring to our scientific community and to the public.”
